This image CAN be licensed for re-use by Kestrel Illustration Studio.
How may I purchase this image for use?
Phophate Starvation
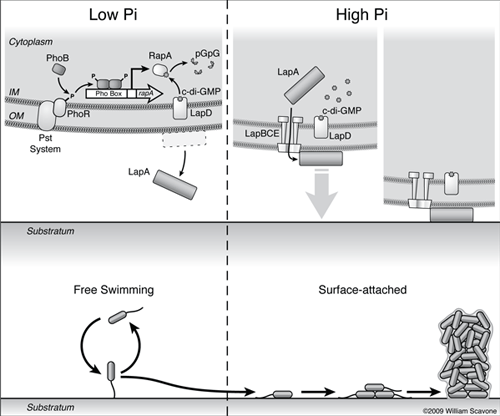
A schematic summarizing the current model for how phosphate starvation represses biofilm formation by Pf (Pseudomonas fluorescens) is shown.
Low extracellar Pi is sensed by the PhoR/Pst system complex, and leads to activation of the PhoR kinase and phosphorylation of PhoB. PhoB~P forms a dimer and binds to the Pho Box sequence upstream of rapA, activating its transcription.
RapA cleaves c-di-GMP (cyclic di-GMP) to form pGpG (linear di-GMP, which is the cleavage product of cdiGMP which has been treated with a phosphodiesterase) through its PDE (phosphodiesterase) activity, depleting cellular c-di-GMP pools.
Decreased c-di-GMP levels block the secretion of the LapA adhesin to the cell surface via an unknown mechanism.
Depletion of c-di-GMP leads to dissociation of the nucleotide from the c-di-GMP effector LapD, and this signal promotes the egress of LapA to the culture supernatant.
On the right, high extracelular phosphate and increased c-di-GMP levels promote the secretion of the LapA adhesin to the cell surface, resulting in surface attachment of Pf and biofilm formation.
Text by George O'Toole, Ph.D. et. al., Department of Microbology and Immunology, Dartmouth Medical School
© William Scavone. All Rights Reserved.
